Some science for the gardener
- This is Section 3 of the Gardening Seminar:Start your own garden. Comments or questions are appreciated. You can also write to John Eagles.
This chapter is unavoidably a bit more complex. I still advise you to spend some time studying these topics as this content is important for many processes of growth in your garden. Understanding the scientific explanation helps you to make the right decisions regarding the plants you grow, how you manage the soil and what fertilizers you need.
The topics of this section of the seminar are:
- Photosynthesis
- The nitrogen cycle
- pH.
Each of these explains about essential conditions that are needed for plants to grow well.
Photosynthesis
- Main article: Photosynthesis
All green plants can be seen as little factories that produce energy and food for all other living beings. The basic elements they need for this are water, air and sunlight. The process that runs in these countless factories is called 'photosynthesis.'
Photosynthesis takes place in a green pigment in plants, so-called chlorophyll, which absorbs sunlight. Plants are green because plants cannot absorb the green light that comes from the sun, though they absorb blue and red light. The green light is reflected back and makes the plant appear green.
Photosynthesis is the process by which green plants and some other organisms use sunlight to synthesize foods from carbon dioxide and water. It is the magical process that makes all our gardening and farming worthwhile. Air (carbondioxide) and water and sunlight are transformed into energy (sugar) and the structural element (carbon) for cells and oxygen to breathe.
Many plants survive in low light conditions. But when a plant is denied enough light, it attempts to grow taller in order to find light. The plant is taller but also weaker.
Photosynthesis is vital for all life on Earth that needs oxygen.
- Photosynthesis maintains normal levels of oxygen in the atmosphere
- It is the source of energy for building up the cells of an organism in which photosynthesis occurs, or as a source of food, it gives this energy to other organisms. For example, humans and animals eat plants.
Nitrogen cycle
- Main article: Nitrogen cycle
The diagram to the right shows how nitrogen gas that is present in the atmosphere goes into a cycle that makes nitrogen available to plants.
- Nitrogen from the air is fixed by bacteria that live as nodules on roots of legumes[1] and some other plant species. These nitrogen-fixing bacteria give nitrogen to the plants on which they live, and when these plants die, the nitrogen remains in the soil.
- Nitrogen from the air is fixed by nitrogen-fixing soil bacteria. They transform nitrogen to ammonium (NH4+). This process is called ammonification[2].
- Animals drop manure into the soil. Animals and plants die and animal waste and dead animals and plants are decomposed by aerobic and anaerobic fungi and bacteria. Also through this process, ammonium (NH4+) is formed.
- Nitrifying bacteria transform ammonium (NH4+) first into nitrites (NO2-) and then into nitrates (NO3-) in a process called nitrification[3].
- Plants can assimilate nitrates (NO3-). Nitrates are also affected by denitrifying bacteria that bring nitrogen gas back into the atmosphere in a process called denitrification[4].
Some practical implications
Plants need nitrogen for their growth. A main component of fertilizers is nitrogen in a form that plants can take in. One way to improve your soil is by adding compost that is rich in nitrogen. Another method is to grow legumes (for example peas and beans, and a few other plant species, not legumes) that form root nodules with nitrogen-fixing bacteria. Yet another method is to sow fast growing plants only for the improvement of your soil. These plants are called 'green manure' plants. Some examples of green manure plants are phacelia, rye and mustard but there are many more. Yet another method is to cover the soil with a layer of decomposing plants, in the form of hay or straw or material of any other plants. This method is called mulching. These plants decompose and enrich the soil with humus-forming components and also with nitrogen.
To enable a speedy process of ammonification and nitrification in your soil, your soil should not only be rich of compost or decomposing plant materials, but should also contain enough oxygen, as most micro-organisms and also animals such as rain worms that decompose these materials need oxygen. By bringing more oxygen into the soil you can also prevent denitrification to take place as denitrifying bacteria operate when there is not enough oxygen in the soil.
Oxygen can be brought into the soil in various ways. First by ploughing or cultivating a field. Second by adding enough organic material, in the form of mulch or compost or decomposed animal manure. Third by planting green manure plants and also plants that have deep roots. When these plants die, their roots leave channels in the soil where air with oxygen can penetrate.
pH
- Main article: PH
The pH of your soil is important because it very much determines how much nutrients will be available to your crops.
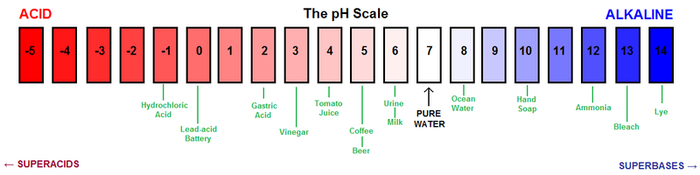
Soil pH is a measure for how many H+ ions a soil contains. pH is a measure of how acidic or basic things are.
pH is defined as the negative logarithm (base 10) of the activity of hydrogen ions (H+) in solution. It ranges from 0 to 14, with 7 being neutral. A pH below 7 is acidic and above 7 is basic.[5] A pH of 6 means that there are 10 times more H+ ions in the soil compared to pH 7, while, for example, a pH of 3 means that there are 10,000(104) times more H+ ions in solution compared to pH 7.
Substances such as lemon juice and battery acid are acidic and fall in the 0-7 range, whereas seawater and bleach are basic (also called "alkaline") and fall in the 7-14 pH range. Pure water is neutral, or 7 on the pH scale.
Soil pH controls many chemical processes that take place in the soil. It specifically affects plant nutrient availability by changing the chemical form in which these nutrients are available. The optimum pH range for most plants is between 6 and 7.5, but many plants have adapted to pH values outside this range.
Classification of soil pH ranges
A peaty soil is acid. Some clay soils are neutral. Chalky soils are alkaline or basic or limey.
Why soils become acidic
Rainfall and leaching
Acidic soils are most often found in areas of high rainfall. In time, excessive rainfall leaches the soil profile's basic elements (calcium, magnesium, sodium, and potassium) that prevent soil acidity. Additionally, rainwater has a slightly acidic pH of 5.7 due to a reaction with CO2 in the atmosphere that forms carbonic acid.
Acidic parent material
Soils that develop from weathered granite (granite is the parent material of most soils in the continental United States [6]) are likely to be more acidic than those developed from shale or limestone.[7] Also soils that contain peat are more acidic.
Organic matter decay
Organic matter decay produces hydrogen ions. This is why it is good to add some lime to your compost heap.
High-yielding crops
- "Harvest of high-yielding crops plays the most significant role in increasing soil acidity. During growth, crops absorb basic elements such as calcium, magnesium, and potassium to satisfy their nutritional requirements. As crop yields increase, more of these limelike nutrients are removed from the field."[8]
How much lime to add to your soil
These data are for ground limestone (garden lime, largely composed of calcium carbonate (CaCO3)):[9]
- Add 270g a sq m (8oz a sq yd) of lime to raise the pH one point in sandy soils.
- Add 540g a sq m (16oz a sq yd) of lime to raise the pH one point in loam soils.
- Add 800g a sq m (24oz a sq yd) of lime to raise the pH one point in clay soils.
Determining soil pH
When a soil looks good, doesn't silt up, has ample good-smelling humus etc. you can be sure that the pH is also in the range of normal.
- You can use an inexpensive pH testing kit based on barium sulphate in powdered form, where in a small sample of soil is mixed with water which changes colour according to the acidity/alkalinity.
- You can use litmus paper. A small sample of soil is mixed with distilled water, into which a strip of litmus paper is inserted. If the soil is acidic the paper turns red, if alkaline, blue.
- You can use a commercially available electronic pH meter, in which a rod is inserted into moistened soil and measures the concentration of hydrogen ions.
- You can also send a sample of your soil to an expert laboratory. To have a test done at a soil laboratory can be helpful as they also give information about nutrients missing or available. For a smaller garden, one test taken from a mix of several samples would be sufficient.
- Finally, you can observe indicator plants for pH in your garden:
- Plants that prefer an acidic soil include Erica (Heathers, Rhododendron and nearly all other Ericaceae species, many Birch (Betula), foxglove (Digitalis), Moss, gorse (Ulex spp.), and Scots Pine (Pinus sylvestris).
- Lime loving plants include Ash trees (Fraxinus spp.), Honeysuckle (Lonicera), Buddleja, dogwoods (Cornus spp.), lilac (Syringa) and Clematis species.[10]
Back
- Gardening Seminar:Start your own garden to go to the other sections of this seminar.
- Designing your garden to go to the former section of this seminar
Next section
See also
References
- ↑ Legumes - Wikipedia "A legume is a plant in the family Fabaceae (or Leguminosae), or a fruit of these specific plants."
- ↑ Ammonification - Wikipedia "When a plant or animal dies, or an animal expels waste, the initial form of nitrogen is organic. Bacteria, or fungi in some cases, convert the organic nitrogen within the remains back into ammonium (NH4+), a process called ammonification or mineralization."
- ↑ Nitrification - Wikipedia "Nitrification is the biological oxidation of ammonia with oxygen into nitrite followed by the oxidation of these nitrites into nitrates."
- ↑ Denitrification - Wikipedia "Denitrification is a microbially facilitated process of nitrate reduction that may ultimately produce molecular nitrogen (N2) through a series of intermediate gaseous nitrogen oxide products."
- ↑ Soil pH - Wikipedia
- ↑ Soil nutrient management for Maui County, University of Hawai'i
- ↑ Understanding and Correcting Soil Acidity by Jeff Ball
- ↑ Understanding and Correcting Soil Acidity by Jeff Ball
- ↑ Which - Gardening - Lime and pH
- ↑ Soil pH - Wikipedia
External links
- pH - Wikipedia
- Photosynthesis - Wikipedia
- Nitrogen cycle - Wikipedia
- Soil pH Wikipedia
- Understanding and Correcting Soil Acidity by Jeff Ball, The Samuel Roberts Noble Foundation
Comments
- Coming soon