Difference between revisions of "PH"
| Line 54: | Line 54: | ||
=== Acidic parent material === | === Acidic parent material === | ||
| − | Soils that develop from weathered | + | Soils that develop from weathered granite (Granite is the parent material of most soils in the continental United States [http://www.ctahr.hawaii.edu/mauisoil/a_factor_form.aspx Soil nutrient management for Maui County, University of Hawai'i]</ref>) are likely to be more acidic than those developed from shale or limestone.<ref>[http://www.noble.org/ag/soils/soilacidity/index.htm Understanding and Correcting Soil Acidity by Jeff Ball ]</ref> Also soils that contain peat are more acidic. |
=== Organic matter decay === | === Organic matter decay === | ||
Revision as of 16:54, 15 January 2012
- Topic in Gardening courses
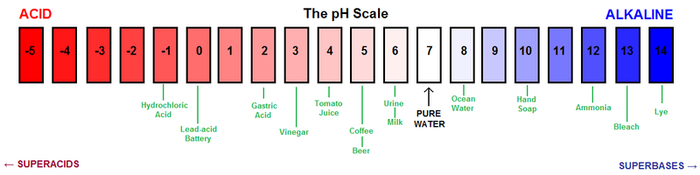
Soil pH is a measure for how many H+ ions a soil contains. pH is a measure of how acidic or basic things are.
pH is defined as the negative logarithm (base 10) of the activity of hydrogen ions (H+) in solution. It ranges from 0 to 14, with 7 being neutral. A pH below 7 is acidic and above 7 is basic.[1] A pH of 6 means that there are 10 times more H+ ions in the soil compared to pH 7, while, for example, a pH of 3 means that there are 10,000(104) times more H+ ions in solution compared to pH 7.
Substances such as lemon juice and battery acid are acidic and fall in the 0-7 range, whereas seawater and bleach are basic (also called "alkaline") and fall in the 7-14 pH range. Pure water is neutral, or 7 on the pH scale.
Soil pH controls many chemical processes that take place in the soil. It specifically affects plant nutrient availability by changing the chemical form in which these nutrients are available. The optimum pH range for most plants is between 6 and 7.5, but many plants have adapted to pH values outside this range.
Classification of soil pH ranges
A peaty soil is acid. Some clay soils are neutral. Chalky soils are alkaline or basic or limey.
| Denomination | pH range |
|---|---|
| Ultra acid | <3.5 |
| Extreme acid | 3.5 - 4.4 |
| Very strong acid | 4.5 - 5.0 |
| Strong acid | 5.1 - 5.5 |
| Moderate acid | 5.6 -6.0 |
| Slight acid | 6.1 -6.5 |
| Neutral | 6.6 - 7.3 |
| Slightly alkaline | 7.4 - 7.8 |
| Moderately alkaline | 7.9 - 8.4 |
| Strongly alkaline | 8.5 -9.0 |
| Very strongly alkaline | >9.0 |
Source: The United States Department of Agriculture Natural Resources Conservation Service
Why soils become acidic
While the pH of a soil is measured based on how many H+ a soil contains, Aluminium ions Al3+ are also important. In acidic soils Al3+ reacts with water and releases extra H+ ions.
Rainfall and leaching
Acidic soils are most often found in areas of high rainfall. In time, excessive rainfall leaches the soil profile's basic elements (calcium, magnesium, sodium, and potassium) that prevent soil acidity. Additionally, rainwater has a slightly acidic pH of 5.7 due to a reaction with CO2 in the atmosphere that forms carbonic acid.
Acidic parent material
Soils that develop from weathered granite (Granite is the parent material of most soils in the continental United States Soil nutrient management for Maui County, University of Hawai'i</ref>) are likely to be more acidic than those developed from shale or limestone.[2] Also soils that contain peat are more acidic.
Organic matter decay
Organic matter decay produces hydrogen ions. This is why it is good to add some lime to your compost heap.
High-yielding crops
- "Harvest of high-yielding crops plays the most significant role in increasing soil acidity. During growth, crops absorb basic elements such as calcium, magnesium, and potassium to satisfy their nutritional requirements. As crop yields increase, more of these limelike nutrients are removed from the field."[3]
How much lime to add to your soil
These data are for ground limestone (garden lime):[4]
- Add 270g a sq m (8oz a sq yd) of lime to raise the pH one point in sandy soils.
- Add 540g a sq m (16oz a sq yd) of lime to raise the pH one point in loam soils.
- Add 800g a sq m (24oz a sq yd) of lime to raise the pH one point in clay soils.
Availability of nutrients in relation to pH
There are 17 essential plant nutrients. Carbon and oxygen are absorbed from the air, while other nutrients including water are obtained from the soil. Plants must obtain the following mineral nutrients from the growing media:
- the primary macronutrients: nitrogen (N), phosphorus (P), potassium (K)
- the three secondary macronutrients: calcium (Ca), sulphur (S), magnesium (Mg)
- the macronutrient Silicon (Si)
- the micronutrients or trace minerals: boron (B), chlorine (Cl), manganese (Mn), iron (Fe), zinc (Zn), copper (Cu), molybdenum (Mo), nickel (Ni), selenium (Se), and sodium (Na).[5]
In slightly to moderately alkaline soils P, Fe, Mn, Zn Cu, and Co levels are reduced so low they may affect plant growth.
In acid soils, micro-nutrient availability (except Mo and Bo) is increased.
Dissolved nitrogen (N) and nitrogen as applied in fertilizer gifts such as ammonium (NH4) or nitrate (NO3) will have the highest concentrations in soil with pH 6-8.
In order for P to be available for plants, soil pH needs to be in the range 6.0 and 7.5. If pH is lower than 6, P starts forming insoluble compounds with iron (Fe) and aluminium (Al) and if pH is higher than 7.5 P starts forming insoluble compounds with calcium (Ca).
In soils that already contain the essential nutrients, most nutrient deficiencies can be avoided between a pH range of 5.5 to 6.5.[6]
Indicator plants for pH
Plant pH preferences
- This list is still under construction and needs to be checked with various sources
- pH 4.5-5.0 Blueberry, Bilberry, Heather, Cranberry, Orchid, Azalea
- pH 5.0 - 5.5 Parsley, Potato, Heather, Pine, Maize, Radish, Ferns, Iris, Rhododendron
- pH 5.5 - 6.0 Bean, Brussels Sprouts, Carrot, Endive, Rhubarb, Soybean, Aster, Begonia, Daffodil, Zucchini, most bulbs
- pH 6.0 - 6.5 Broccoli, Cauliflower, Cucumber, Egg Plant, Hemp, Pea, Pumpkin, Tomato, Turnip, Clover, Gladiolus, Poppy, Rose, Viola, Wallflower, Zinnea, Strawberry
- pH 6.5 - 7.0 Asparagus, Beetroot , Cabbage, Celery, Lettuce, Melons, Onion, Parsnip, Spinach, Alfalfa, Chrysanthemum, Dahlia, Sweet Pea and Tulip.
- pH 7.1 - 8.0 Lilac, brassica
See also
References
External links
- Soil pH Wikipedia
- Understanding and Correcting Soil Acidity by Jeff Ball, The Samuel Roberts Noble Foundation